Research
My research primarily focuses on engineering biomaterials for localized disease management across various applications. Currently at CMU I employ synthetic tools to design a variety of materials such as soft hydrogels to robust bone cements spanning a range of biomedical applications. My current research efforts at CMU focus on the following research problems:
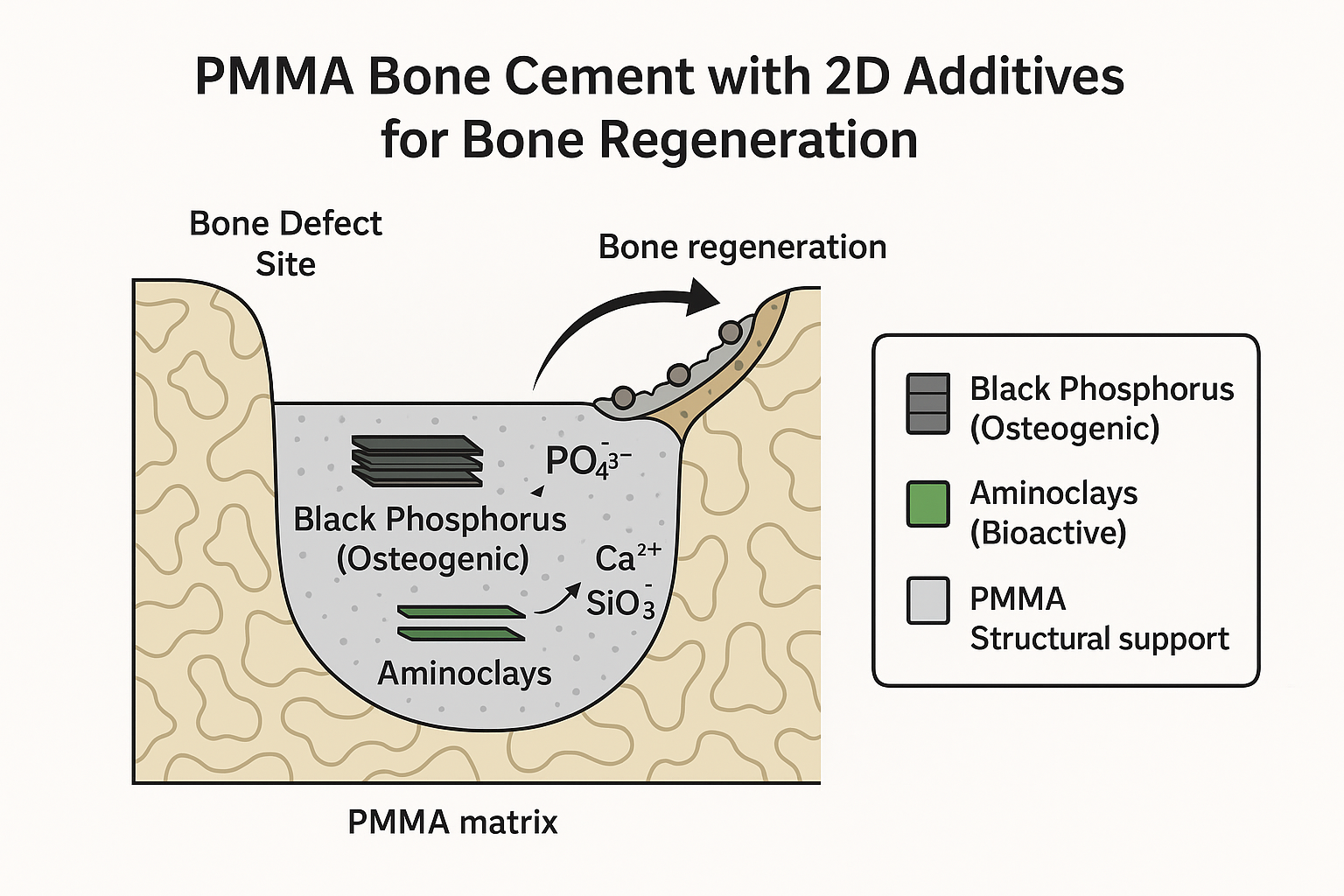
Bone Regeneration and Prosthetic Infections Prevention
- Bone regeneration and prevention of prosthetic joint infections remain major clinical challenges, particularly in cases of osteomyelitis and revision surgeries. Conventional PMMA bone cements lack bioactivity and antimicrobial properties, leading to poor bone integration and infection risks.
My Approach:
- Enhancing bone regeneration through bioactive 2D nanomaterials (aminoclays, black phosphorus) incorporated into PMMA bone cements to improve osteoconductivity.
- Preventing infections by integrating antibiotic-functionalized co-monomers and localized drug delivery strategies for long-term antimicrobial activity without compromising mechanical strength.
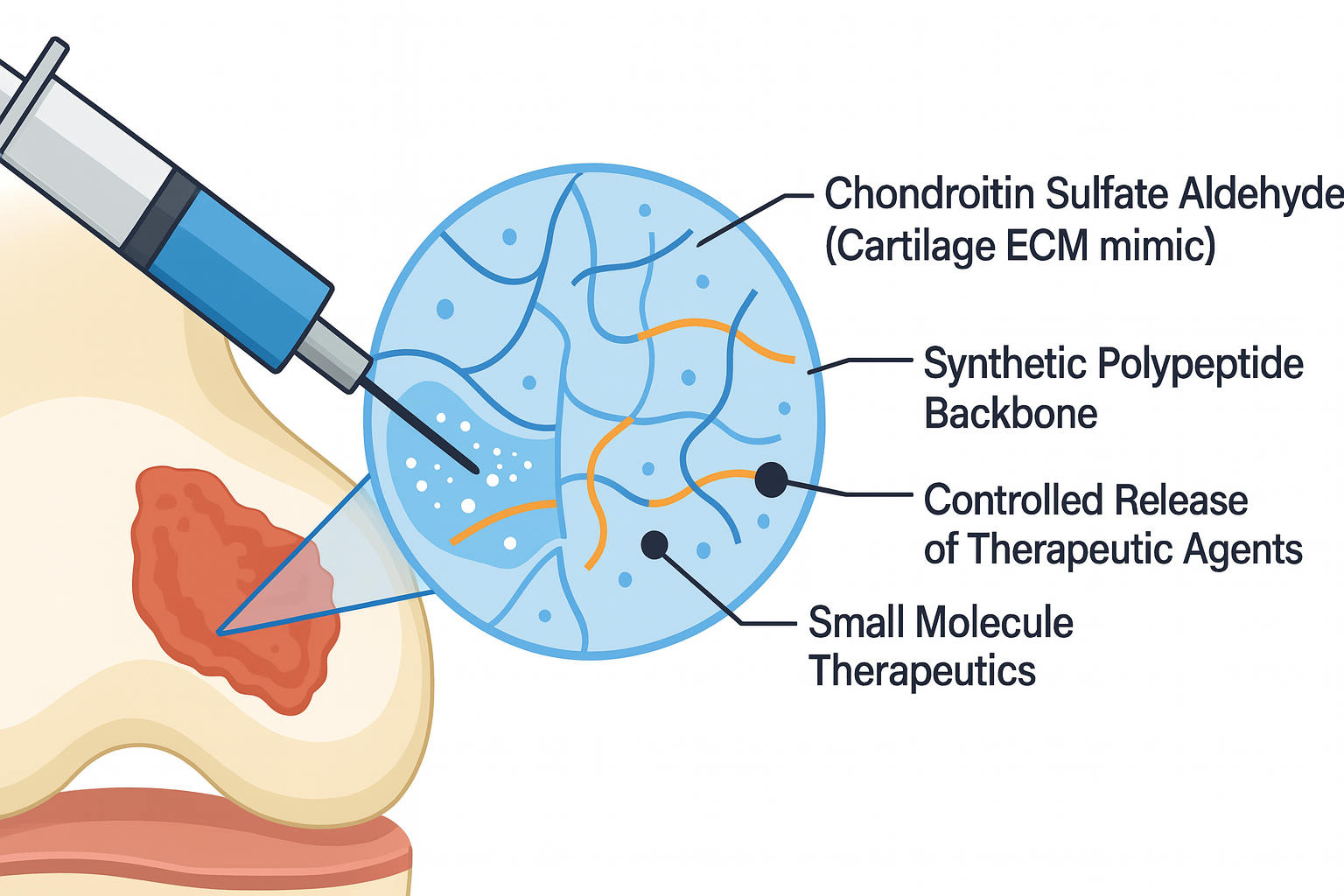
Cartilage Mimic Hydrogels for Cartilage Regeneration
- Cartilage has minimal regenerative capacity, and in osteoarthritis, progressive cartilage degradation leads to bone-on-bone contact, chronic inflammation, and joint degeneration. Existing treatment options fail to restore native cartilage structure and mechanics.
My Approach:
- Designing injectable hydrogels composed of Chondroitin Sulfate and synthetic polypeptides that mimic the native cartilage not only in terms of chemical composition but mechanical properties.
- Incorporating chemical cues in form of short peptides or small molecule drugs to promote chondrogenesis at cartilage defect site.
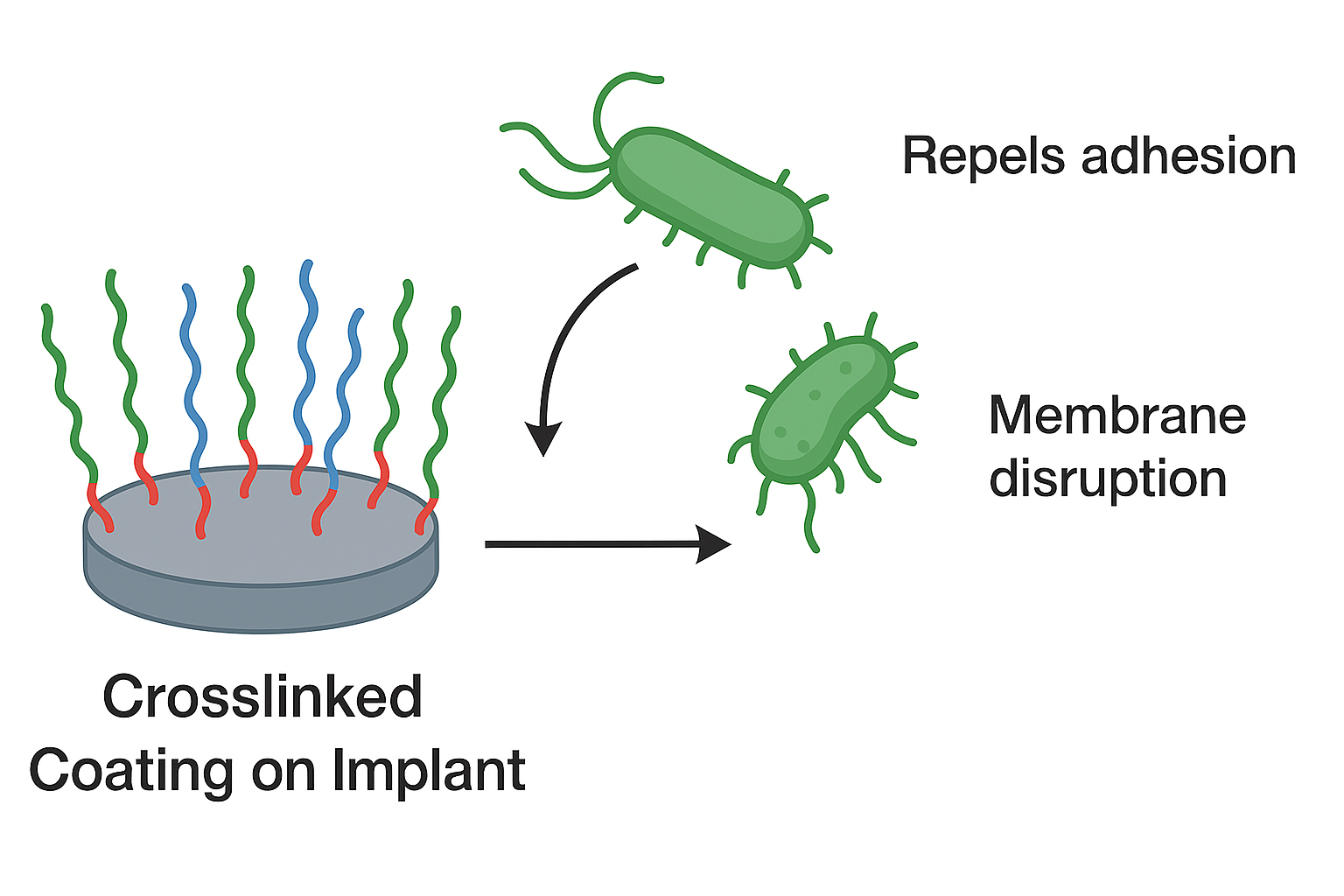
Synthetic Polypeptides as Antimicrobial Coatings
- Microbial adhesion and biofilm formation on surfaces cause infections in healthcare and contamination in food and industrial systems. Existing solutions—antibiotics, disinfectants, and passive coatings—offer only short-term protection and face issues like resistance, toxicity, and performance loss. There is a critical need for durable, biocompatible antimicrobial coatings that provide long-lasting protection across applications.
My Approach:
-
I focus on developing advanced surface and material solutions to reduce microbial adhesion and contamination across critical sectors, including healthcare, food processing, and industrial systems. The goal is to create durable, biocompatible, and scalable coatings that:
- Minimize microbial colonization to lower infection and contamination risks.
- Maintain long-term surface functionality under dynamic environmental conditions.
- Enable safe, application-specific customization without compromising structural or operational integrity.
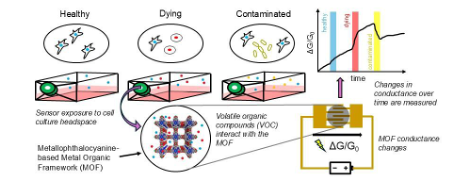
Electroanalytical Platform for Mammalian Culture Health Assessment
- Cartilage has minimal regenerative capacity, and in osteoarthritis, progressive cartilage degradation leads to bone-on-bone contact, chronic inflammation, and joint degeneration. Existing treatment options fail to restore native cartilage structure and mechanics.
My Approach:
- Designing injectable hydrogels composed of Chondroitin Sulfate and synthetic polypeptides that mimic the native cartilage not only in terms of chemical composition but mechanical properties.
- Incorporating chemical cues in form of short peptides or small molecule drugs to promote chondrogenesis at cartilage defect site.